A Bold Step Forward: Donanemab’s Promise and Challenges
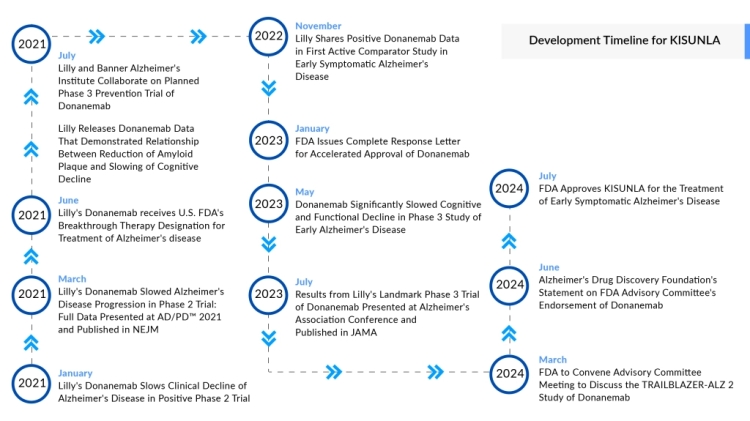
A Significant Advancement in Alzheimer’s Care
The FDA has officially approved Eli Lilly’s donanemab, marking a major step forward in the treatment of Alzheimer’s disease. This monoclonal antibody therapy is designed to target and clear amyloid plaques, a key contributor to disease progression. With this approval, Eli Lilly strengthens its position in the Alzheimer’s market, bringing new hope to patients and their families.
Donanemab vs. Lecanemab: Key Differences
The TRAILBLAZER-ALZ 3 trial demonstrated that donanemab effectively slows cognitive decline in patients with early-stage Alzheimer’s. A comparison of donanemab vs. lecanemab shows that both therapies achieve similar reductions in amyloid buildup, but they differ in dosing schedules and safety profiles. These differences may influence prescribing decisions among healthcare providers.
The Approval Process and Safety Concerns
The donanemab approval process involved extensive regulatory review, particularly regarding the risk of amyloid-related imaging abnormalities (ARIA). While safety concerns were raised, the FDA determined that donanemab’s benefits in slowing disease progression outweigh the potential risks, leading to its approval.
The Evolving Landscape of Alzheimer’s Treatment
As new therapies enter the market, the field of Alzheimer’s treatment continues to advance. With donanemab now available, discussions on pricing, insurance coverage, and accessibility will play a crucial role in determining its reach. This approval represents a major step forward in Alzheimer’s care and highlights the potential for future innovations in the fight against the disease.
Latest Reports Offered By Delveinsight
Spinocerebellar Ataxia Market | Tenosynovitis Market | Thymic Carcinoma Market | Trichomoniasis Market | Tuberculous Meningitis Market | Typhoid Fever Market | Uremic Pruritus Market | Usher Syndrome Market | Vertebral Body Replacement Systems Market | Von Willebrand Disease Market | Warm Autoimmune Hemolytic Anemia Market | Warts Market | Wegener's Granulomatosis Market | Acute Pancreatitis Market | Acute Respiratory Distress Syndrome Market | Adrenogenital Syndrome Market | Alcoholic Hepatitis Market | Amyloidosis Market | Angioedema Market | Ankylosing Spondylitis Market | Atypical Hemolytic Uremic Syndrome (aHUS) Market | B-Cell Maturation Antigen Targeted Therapies Market | Biliary Tumor Market | Cognitive Impairment Associated With Schizophrenia Market
About DelveInsight
DelveInsight is a market research and consulting firm specializing in life sciences and healthcare. We deliver valuable insights to help pharmaceutical, biotechnology, and medical device companies succeed in a competitive and rapidly changing industry.
Contact Information
Kanishk
Email: kkumar@delveinsight.com
What's Your Reaction?
















.jpg)
.jpg)


